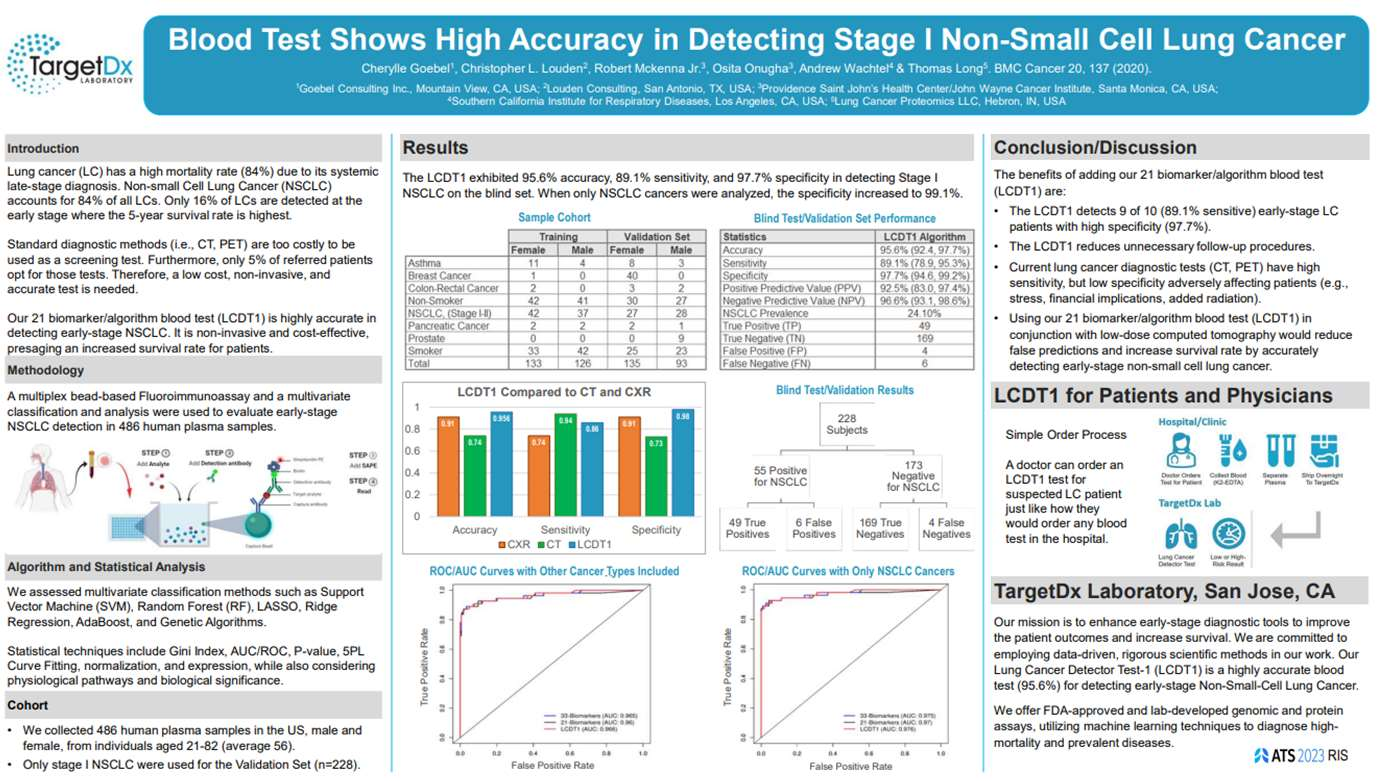
The LCDT1 is a simple blood test that allows for detection of early-stage Non-Small Cell Lung Cancer (NSCLC). This device is expected to have an accuracy of 95.6%, a sensitivity of 89.1%, and a specificity of 97.7%. Performance and results have been validated and published. The LCDT1 is patented and proprietary.
Click on the image to display it in full size:
Biomarkers serve as essential health indicators, reflecting the interplay between genetic expression and environmental factors within your body. Commonly measured through substances like proteins, DNA, or hormones, these markers are found in tissues and bodily fluids and provide insights into both normal physiological processes and potential disease states.
Our LCDT1 technology harnesses the specificity of protein biomarkers for several key reasons:
The LCDT1 algorithm by TargetDx represents an advanced approach to assessing an individual's risk for lung cancer. This method involves analyzing protein biomarkers within a plasma sample using machine learning techniques. Machine learning is a form of artificial intelligence that enables computers to learn from data, identifying patterns and making decisions with minimal human intervention.
At TargetDx, the proprietary algorithm is designed to integrate specific protein biomarker data with relevant patient demographics. The algorithm processes this information to predict the likelihood of early-stage non-small cell lung cancer (NSCLC). The outcome of the analysis is a categorized risk level, indicating whether a patient is at high risk or low risk for NSCLC, facilitating early and potentially life-saving interventions.
The LCDT1 is for individuals who are at risk for lung cancer:
Your physician can order the test if they suspect lung cancer. You will get your blood drawn at the hospital lab and the plasma shipped overnight to our CLIA laboratory. The plasma sample is evaluated for 21 biomarkers using our proprietary algorithm. The results will provide a risk assessment for lung cancer which will be sent to your doctor who will discuss your results and subsequent steps with you.
Blood test shows high accuracy in detecting stage I non-small cell lung cancer
Diagnosis of Non-small Cell Lung Cancer for Early-Stage Asymptomatic Patients
Abstract 1733: Development of a novel blood plasma protein test for diagnosis of lung cancer
Plasma Biomarkers Distinguish Non-small Cell Lung Cancer from Asthma and Differ in Men and Women
PP 9 Men and women display different proteomic diagnostic profiles in non small cell lung cancer
1424 POSTER Methods of Identification and Diagnosis of Lung Cancer Using Classification Systems
79p Mass Spectrometric Identification Of Novel Proteomic Biomarkers Of Non-Small Cell Lung Cancer
Abstract C15: A novel panel of serum biomarkers distinguishes asthma from non‐small cell lung cancer
Serum Biomarkers Of Natural Product Therapy In Human Lung Cancer Xenograft Models
TargetDx Laboratory has secured a robust portfolio of intellectual property surrounding the utilization of protein biomarkers that are pivotal in the pathways associated with lung cancer inflammation. Our suite of global patents includes 31 granted and 16 pending, which encompass advanced methods for analyzing human biological fluids in concert with targeted protein biomarkers using sophisticated machine learning technology. This strategic accumulation of patents places us at the forefront of the lung cancer diagnostics field, with the potential to significantly shape and lead the market.